Cannabinoids, a diverse group of chemical compounds found in cannabis and hemp plants, have gained significant attention for their potential therapeutic benefits. These naturally occurring compounds interact with the endocannabinoid system (ECS) in the human body, regulating various physiological processes. In this blog post, we will delve into the world of cannabinoids, exploring what they are, different types of phytocannabinoids, and the functioning of the ECS.
What are Cannabinoids?
Cannabinoids are a class of chemical compounds that interact with the ECS, a complex signaling system responsible for maintaining homeostasis and regulating various bodily functions. These compounds can be further categorized into two main types: endocannabinoids, which are produced by the human body, and phytocannabinoids, which are found in cannabis and hemp plants. Cannabinoids are primarily derived from the trichomes, the resin-producing glands of cannabis and hemp plants. These trichomes contain a wealth of bioactive compounds, including the diverse array of cannabinoids.
Types of Phytocannabinoids:
Cannabis and hemp plants are abundant sources of phytocannabinoids. Among the numerous cannabinoids identified in these plants, several stand out for their unique properties:
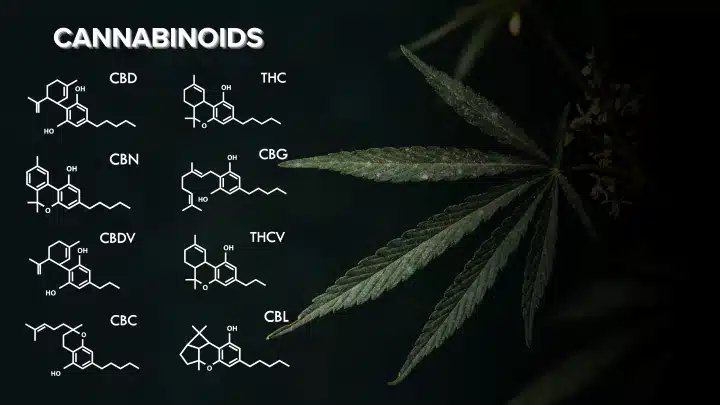
- Cannabichromene (CBC): CBC is a non-psychoactive cannabinoid that may possess anti-inflammatory properties and potential therapeutic benefits.
- Cannabidiol (CBD): CBD is a well-known non-psychoactive cannabinoid with a wide range of potential health benefits, including anxiety reduction, pain relief, and anti-seizure properties.
- Cannabidiolic Acid (CBDa): CBDa is the acidic precursor to CBD and may have anti-inflammatory and anti-nausea properties.
- Cannabigerol (CBG): Known as the “mother cannabinoid,” CBG is a precursor to other cannabinoids and has shown promise as an anti-inflammatory and potential neuroprotectant.
- Cannabigerolic Acid (CBGa): CBGa is the acidic form of CBG and serves as the starting point for the synthesis of various cannabinoids in the plant.
- Cannabinol (CBN): CBN is a product of THC degradation and has potential sedative properties.
- Tetrahydrocannabinol (THC): THC is the primary psychoactive compound in cannabis, responsible for the euphoric “high” experienced with marijuana use. It also exhibits potential therapeutic effects such as pain relief and appetite stimulation.
- Tetrahydrocannabivarin (THCV): THCV is structurally similar to THC but has different effects. It may have some psychoactive properties while also potentially suppressing appetite.
The Endocannabinoid System (ECS):
The ECS is a complex network of receptors, endocannabinoids, and enzymes that play a vital role in maintaining the body’s internal balance. Cannabinoid receptors, CB1 and CB2, interact with phytocannabinoids to produce various effects. Endocannabinoids are endogenous cannabinoids, meaning they are cannabinoids produced within the human body. These lipid-based molecules act as neurotransmitters, facilitating communication between cells and helping to regulate various physiological processes. Among the endocannabinoids identified so far, two of the most well-known and studied are anandamide and 2-arachidonoylglycerol (2-AG). The two primary enzymes, fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), are responsible for synthesizing and breaking down endocannabinoids to maintain ECS balance.
Cannabinoid Receptors:
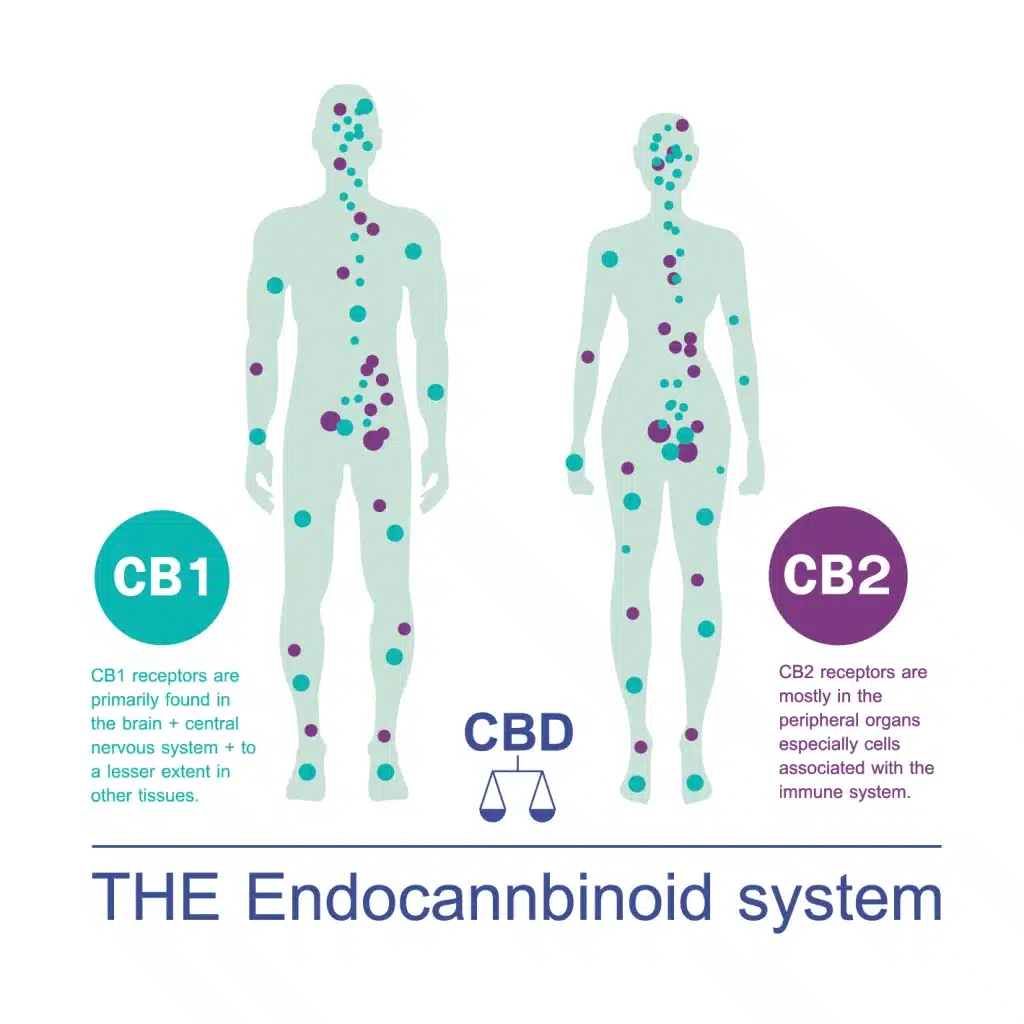
CB1 Receptors: Primarily found in the brain and central nervous system, CB1 receptors play a crucial role in regulating mood, pain, and motor function. THC has a strong affinity for CB1 receptors, leading to its psychoactive effects.
CB2 Receptors: Predominantly located in the immune system and peripheral tissues, CB2 receptors are associated with the regulation of inflammation and immune response. CBD and other cannabinoids interact with CB2 receptors, potentially contributing to their anti-inflammatory effects.
Endocannabinoids:
Anandamide, often referred to as the “bliss molecule,” was the first endocannabinoid to be discovered. Its name is derived from the Sanskrit word “ananda,” which means bliss or joy, due to its potential role in promoting a sense of well-being and happiness. Anandamide primarily binds to cannabinoid receptors in the brain and nervous system, specifically CB1 receptors. This allows it to play a large part in mood regulation, pain perception, and appetite control.
2-Arachidonoylglycerol (2-AG) is another significant endocannabinoid found in the human body. It is more abundant than anandamide and acts on both CB1 and CB2 receptors, although its effects are more pronounced on CB1 receptors in the brain. It has been implicated to function in neuroprotection, immune regulation, and neurotransmitter release.
Enzymes:
Fatty Acid Amide Hydrolase (FAAH) is an enzyme responsible for breaking down anandamide after it fulfills its role and is no longer needed. FAAH comes into action to break it down into its components: arachidonic acid and ethanolamine. This process helps prevent excessive activation of cannabinoid receptors, ensuring that the endocannabinoid system remains balanced. Inhibiting FAAH activity can lead to an increase in anandamide levels, potentially enhancing its therapeutic effects. For this reason, FAAH inhibitors are an area of interest in cannabinoid research.
Monoacylglycerol Lipase (MAGL) is an enzyme responsible for breaking down 2-arachidonoylglycerol (2-AG). MAGL breaks down 2-AG into arachidonic acid and glycerol. By doing so, MAGL ensures that 2-AG levels are tightly regulated within the body. This precise control is essential to maintain the proper functioning of the endocannabinoid system and prevent overactivation of cannabinoid receptors. Similar to FAAH, inhibiting MAGL activity can lead to an increase in 2-AG levels, potentially enhancing its therapeutic effects.
Cannabinoids offer a fascinating and diverse range of compounds that have the potential to impact human health positively. From the non-psychoactive CBD and CBG to the psychoactive THC and the lesser-known CBC, CBDa, CBGa, CBN, and THCV, each cannabinoid presents unique properties and potential therapeutic benefits. As we continue exploring cannabinoids and their interactions within the ECS, we will uncover more insights into their mechanisms of action and potential applications in various medical conditions.
References:
Di Marzo, V., & Piscitelli, F. (2015). The endocannabinoid system and its modulation by phytocannabinoids. Neurotherapeutics, 12(4), 692-698.
Mechoulam, R., Parker, L. A., & Gallily, R. (2002). Cannabidiol: an overview of some pharmacological aspects. Journal of Clinical Pharmacology, 42(S1), 11S-19S.
Borrelli, F., Pagano, E., Romano, B., Panzera, S., Maiello, F., Coppola, D., … & Izzo, A. A. (2014). Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a Cannabis-derived non-psychotropic cannabinoid. Carcinogenesis, 35(12), 2787-2797.
Fine, P. G., & Rosenfeld, M. J. (2013). The endocannabinoid system, cannabinoids, and pain. Rambam Maimonides Medical Journal, 4(4), e0022.
Pertwee, R. G. (2008). The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol, and Δ9-tetrahydrocannabivarin. British Journal of Pharmacology, 153(2), 199-215.
Blankman, J. L., & Cravatt, B. F. (2013). Chemical probes of endocannabinoid metabolism. Pharmacological Reviews, 65(2), 849-871.
Ahn, K., Johnson, D. S., Cravatt, B. F., & McKinney, M. K. (2009). Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chemical Reviews, 109(4), 1885-1904.
Long, J. Z., Li, W., Booker, L., Burston, J. J., Kinsey, S. G., Schlosburg, J. E., … & Cravatt, B. F. (2009). Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nature Chemical Biology, 5(1), 37-44.
Hill, M. N., & Tasker, J. G. (2012). Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic-pituitary-adrenal axis. Neuroscience, 204, 5-16.
Piomelli, D. (2003). The molecular logic of endocannabinoid signalling. Nature Reviews Neuroscience, 4(11), 873-884.
Morales, P., & Reggio, P. H. (2019). An update on non-CB1, non-CB2 cannabinoid related G-protein-coupled receptors. Cannabis and Cannabinoid Research, 4(4), 265-274.
De Petrocellis, L., Ligresti, A., Moriello, A. S., Allarà, M., Bisogno, T., Petrosino, S., … & Di Marzo, V. (2011). Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. British Journal of Pharmacology, 163(7), 1479-1494.
Hurd, Y. L., Yoon, M., Manini, A. F., Hernandez, S., Olmedo, R., Ostman, M., & Jutras-Aswad, D. (2015). Early phase in the development of Cannabidiol as a treatment for addiction: Opioid relapse takes initial center stage. Neurotherapeutics, 12(4), 807-815.
De Long, G. T., Wolf, C. E., Poklis, J. L., Lichtman, A. H., & Poklis, A. (2010). Pharmacological evaluation of the natural constituent of Cannabis sativa, cannabichromene and its modulation by Δ9-tetrahydrocannabinol. Drug and Alcohol Dependence, 112(1-2), 126-133.
Maione, S., Piscitelli, F., Gatta, L., Vita, D., De Petrocellis, L., Palazzo, E., … & Di Marzo, V. (2011). Non-psychoactive cannabinoids modulate the descending pathway of antinociception in anaesthetized rats through several mechanisms of action. British Journal of Pharmacology, 162(3), 584-596.
Russo EB. (2011). Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. British Journal of Pharmacology, 163(7), 1344-1364.
Howlett AC, et al. (2002). International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacological Reviews, 54(2), 161-202.